8+ Which Intervals Are Affected By The Addition Of A Catalyst
Before catalyst is above after catalyst is below C3H8 O2 - CO2 H2O when correctly. Add 05 g of a catalyst to the flask put the bung back into the flask and start the stopwatch.

Hybrid Materials Based On Keggin Phosphotungstate And Bipyridine With Valuable Hydrophobic And Redox Properties Inorganic Chemistry
The following aspects of a reaction affected by the addition of.

. Catalysts speed up chemical reactions. 10Given the balanced equation. In summary -catalysts increase the rate of.
Which Intervals Are Affected By The Addition Of A Catalyst. Which intervals are affected by the addition of a catalyst. Which intervals are affected by the addition of a catalyst.
For example if we have a reaction. Predict how the addition of a catalyst would affect the rate of the reaction below and explain your prediction H2 g I2 g yields 2 HI - 8555041. Which statement describes the energy of the particles in this sample during interval DE.
This is really because the reaction. Although catalysts have no effect on the position of equilibriumiethe yield of the reaction they do allow equilibrium to be reached more quickly. May 26 2014 Rate of reaction can be increased by using a catalyst.
A catalyst has no effect on the position of equilibrium it only speeds up the rate of achieving equilibrium. Which reaction diagram shows the effect of using the appropriate catalyst in a chemical reaction. Adding a catalyst will decrease the activation energy both in the forward direction and the backward direction.
The surface area to volume ratio of the catalyst may affect the. Adding a catalyst to a chemical reaction changes the rate of reaction by causing. Measure 05 g of a catalyst.
Several transition metals can act as catalysts. 1 1 and 2 2 1 and 3 3 2 and 4 4 3 and 4 5 Given the potential energy diagram for a reversible chemical reaction. Which intervals are affected by the addition of a catalyst.
Interval C in this potential energy diagram could be changed by adding a _____. Which intervals are affected by the. The equilibrium constant is the ratio of forward.
1 Both potential energy and average kinetic energy increase. When this happens the rate of the reaction will increase both in the. Aa catalyst Ban indicator Celectrical energy Dthermal energy 5The activation energy of a chemical reaction can be.
Only very minute quantities of the catalyst are required to produce a dramatic change in the rate of the reaction. A I and 2 C 2 and4 B land 3 D 3 and 4. A catalyst lowers the activation energy for example by providing a surface for the reaction to occur on.

Clay Supported Metal Oxide Nanoparticles In Catalytic Advanced Oxidation Processes A Review Abstract Europe Pmc

Metal Nitrate Catalyzed One Pot Oxidative Esterification Of Benzaldehyde With Hydrogen Peroxide In Alcoholic Solutions At Room Temperature New Journal Of Chemistry Rsc Publishing Doi 10 1039 D0nj05671e

Understanding Kinetically Interplaying Reverse Water Gas Shift And Fischer Tropsch Synthesis During Co2 Hydrogenation Over Fe Based Catalysts Sciencedirect

Universe August 2022 Browse Articles

Catalysts November 2018 Browse Articles

Visible Light Driven Methane Conversion With Oxygen Enabled By Atomically Precise Nickel Catalyst Ccs Chemistry
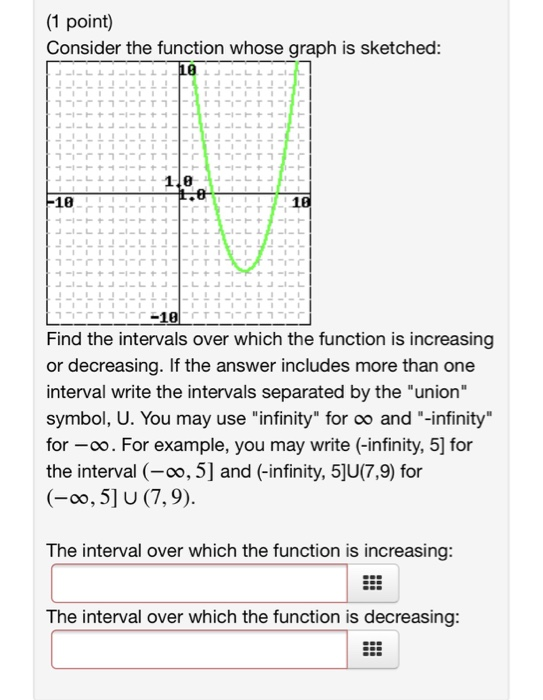
Solved Find The Intervals Over Which The Function Is Chegg Com
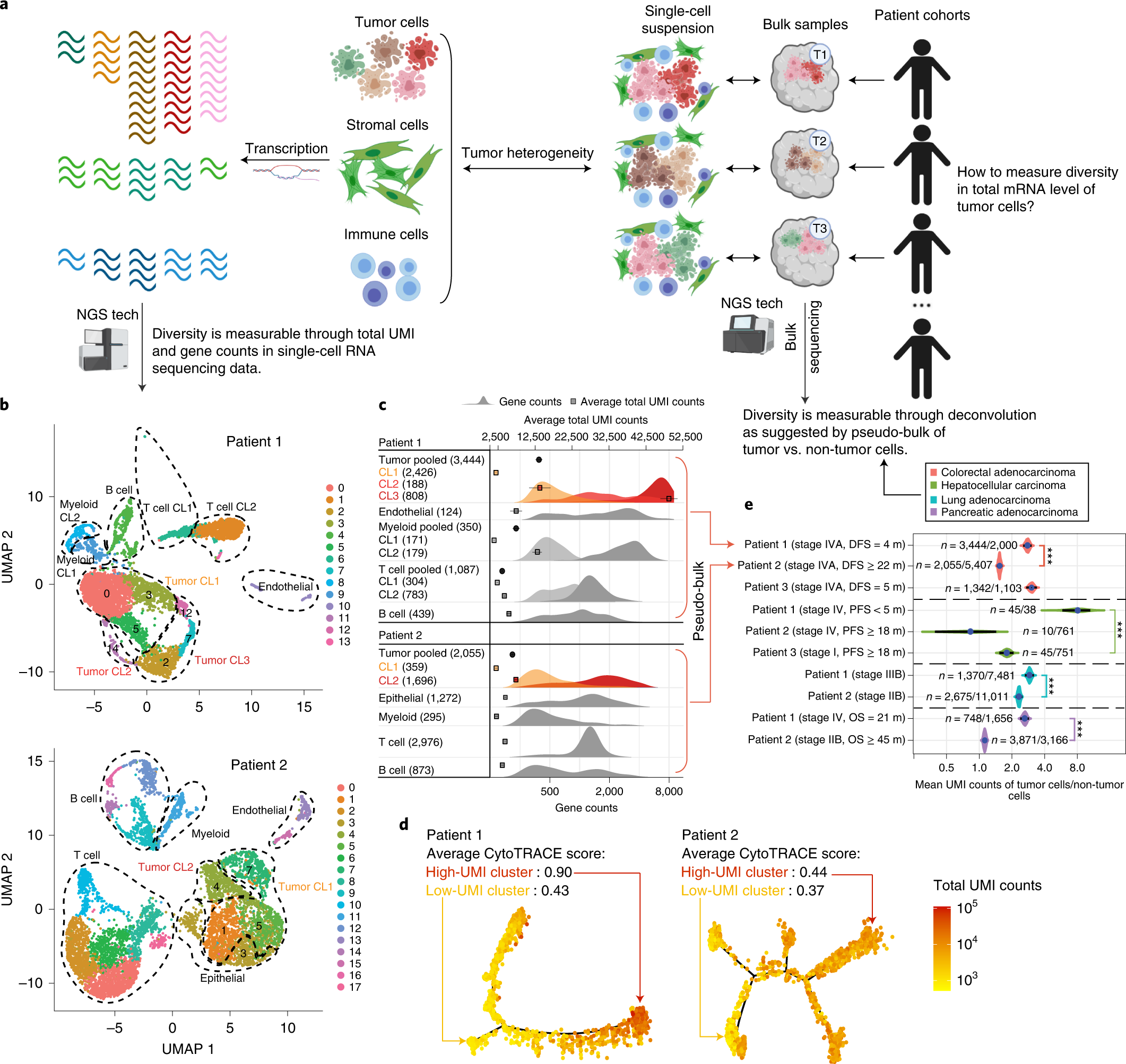
Estimation Of Tumor Cell Total Mrna Expression In 15 Cancer Types Predicts Disease Progression Nature Biotechnology

Structure And Energetics Of Microscopically Inhomogeneous Nanoplasmas In Exploding Clusters
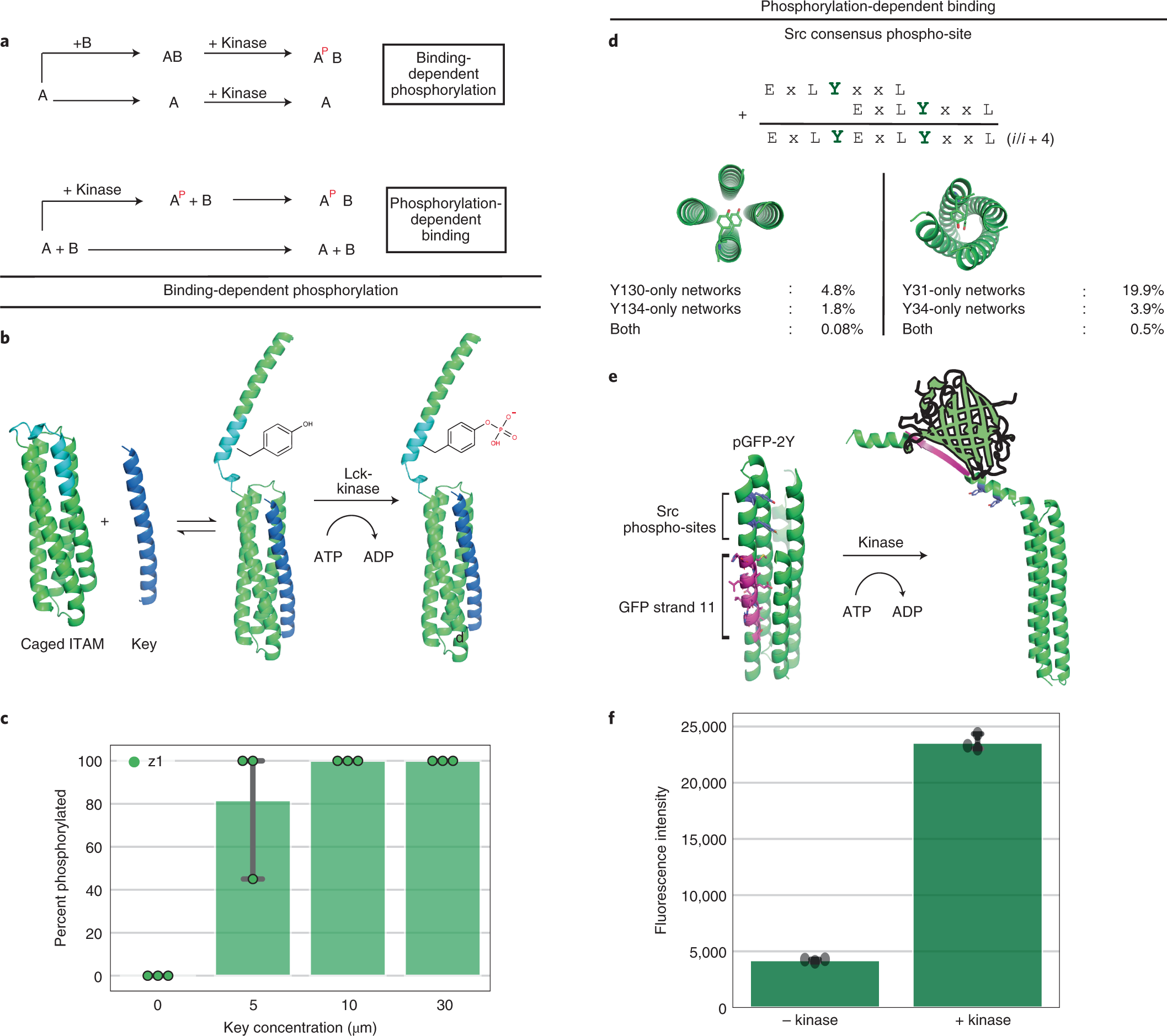
De Novo Design Of Tyrosine And Serine Kinase Driven Protein Switches Nature Structural Molecular Biology

Cisco Content Hub Overview Of The Nam Traffic Analyzer

Pdf Boosted Sono Oxidative Catalytic Degradation Of Brilliant Green Dye By Magnetic Mgfe2o4 Catalyst Degradation Mechanism Assessment Of Bio Toxicity And Cost Analysis
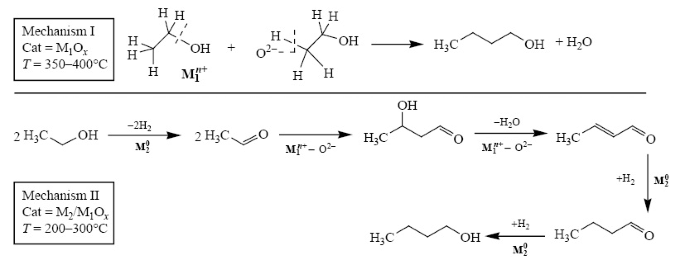
Deactivation Mechanism Of Palladium Catalysts For Ethanol Conversion To Butanol Springerlink

Calculus And Analytic Geometry Thomas G B Finney R J Weir M D

Production Of Aromatics From Butanol Over Ga Promoted Hzsm5 Catalysts Tuning Of Benzene Toluene Xylene And Ethylbenzene Btex Selectivity Reaction Chemistry Engineering Rsc Publishing Doi 10 1039 D1re00531f

Calculus

Chiral Functionalization Of A Zirconium Metal Organic Framework Dut 67 As A Heterogeneous Catalyst In Asymmetric Michael Addition Reaction Inorganic Chemistry